Doubting safety of Covaxin, a native vaccine against Covid19, and double standards for phyto-medicine in the use of biotechnology
On this historic day, the 3rd January 2021, the Drug Controller General of India has given an emergency approval to two vaccines, Covishield of Oxford-AstraZeneca and Covaxin of Bharat Biotech-NIV-ICMR to fight the Covid19 coronavirus that has caused pandemic (https://pib.gov.in/PressReleasePage.aspx?PRID=1685761). With approval, there is a sense of relief amongst people as this virus Co-V2 has so far infected more than 10 million and caused deaths of about 1,46,000 so far in India and a lot more world-wide within a short time of one year. Covishield is also approved in UK after satisfactory results of 3rd phase of trials involving more than 26,000 volunteers in many countries of the Europe and also in India.
Covaxin, an
indigenous vaccine is developed using inactivated virus which still retains its
ability to develop antibodies inside the body to fight the live viral infection,
as and when that occurs. This approach to develop vaccine is time-tested as it
has been done for many others like for hepatitis, polio etc. in the past. Covaxin
entered into the 3rd phase of trial in mid-November 2020 with 23000 volunteers, less than targetted 26000; and this number of volunteers remained lower than that
for Covishield, as at some places, there were not enough.
This has
given a stick for the opposition to create the doubts about the safety of
Covaxin. The Samajwadi Party (SP) leader, Akhilesh Yadav called it a BJP vaccine
and like BJP telling lies, he felt that this vaccine cannot be trusted. So was
the case with Shri Shashi Tharoor, Member of Parliament belonging to Indian
National Congress, who questioned unnecessary haste in granting an approval in
view of limited number of volunteers for incomplete duration, and with limited
results. One SP leader also feared that this vaccine might be an attempt to
reduce population by the party in governance. Thus, politics soon cast a
shadow over the good news of vaccine approval.
Apart from
trials on animals, vaccines being medicine directly applied topically or
injected into humans are to be tested on a large scale. Safety and efficacy is
the must. In case of vaccine, immunogenicity in terms of durational efficacy is
an additional consideration. Mr Harsh Vardhan, Health Minister repeatedly said
that there is no compromise on these criteria. And as Covishield is produced in
about 5 million doses by our Serum Institute of India (SII), Pune; this will be
first administered to those in priority such as health workers. India is in unique position, as it has been a
largest producer of vaccines in the world. With license from
Oxford-AstraZeneca, Covishield already produced by SII in expectation of
emergency approval is reasonably priced. And some states will provide it to the
people free of cost. Soon to follow will be Covaxin of Bharat Biotech which is
being produced in large numbers and made available at reasonable price.
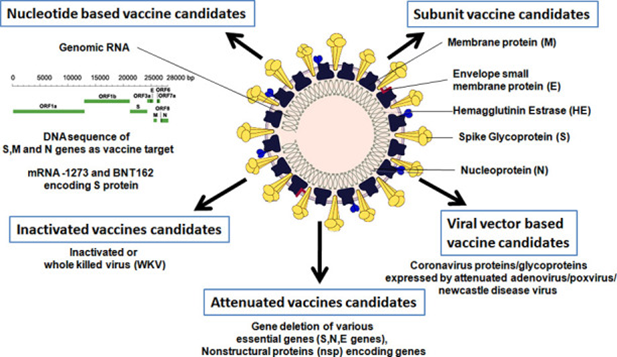
Normally,
vaccine development takes many years, but considering the nature of disease on
a pandemic scale, vaccines are developed within a short period as the knowledge
of developing medicines has improved along with technologies associated (see
below photo from Pandey et al. 2020 Life Sci. https://dx.doi.org/10.1016%2Fj.lfs.2020.117956).
Fig credit:
Pandey et al. 2020 as above
Controversy
over vaccine is also due to fears of the side effects rather than the
benefits of main effects of vaccine. Allergic reactions are common and
differ from person to person and over time in the same person. Most of these
reactions are temporary lasting for hours at the most. Even in daily lives, we
come across allergic reaction, sometime to the food or its contents despite the
fact we might be eating it for many days, or months or even years. Recently, I
observed eating of pickles is causing rashes, that was not the case in the
past. Exposing skin to the sunshine in this winter showed a part of skin
showing redness followed by itching. These allergic reactions last for few
hours. It is heard that only some allergies prove fatal to few people.
This
controversy with political twist reminds me of safety tests required for
development of pesticides, often called phyto-medicines that protect crops
against damage of pests. Safety tests or toxicological tests are carried out as
per protocol before approval of these phyto-medicine by the regulatory
authorities. Efficacy and safety are the main considerations hereto for
approval just like for vaccine or therapeuticals. Most environmental concerns
are addressed along especially for phyto-medicine, if not so rigorously for
human-medicines. Yet, some often criticise as they feel that these tests are
not tried on a long term basis or over generations in the test animals, despite
the fact that these tests are developed in harmony based upon the knowledge and
past experience. I see some parallel here but tweaking for human medicine.
Are there
double standards being used in the development of medicines by the regulatory
authorities? …… in the name of pandemic nature of disease or need for exigency
to find cure? I do not mean, it should be business as usual for human medicine, neither I bother about few hundred less or more when testing is done in thousands. At the same time, certainly, for phyto-medicines too, it should be time-bound
processing for approval, as food security is of equally great concern. Only
on ensuring food security, we will be ensuring human sustenance. Further,
biotechnology use in human medicine development, and in this case for
Covishield vaccine, is acceptable to the regulatory authorities even in Europe.
This technology has brought in a revolution in terms of efficacy, efficiency
and accuracy, for development of products of importance to humans. However, in
case of phyto-medicines, biotechnology or genetic engineering is not acceptable
to the regulatory authorities de facto, if not de jure, no matter
how important these and other scientifically proven technologies are for crop improvement
and protection.
It is time
to look forward to the use of technology for humankind.



Comments
Post a Comment